The COSTED Trial (Cessation Of Smoking Trial in the Emergency Department) results are in and it shows vaping in a very positive light!

You can read the full report here: Cessation of Smoking Trial in the Emergency Department (COSTED): a multicentre randomised controlled trial.
About The COSTED Trial
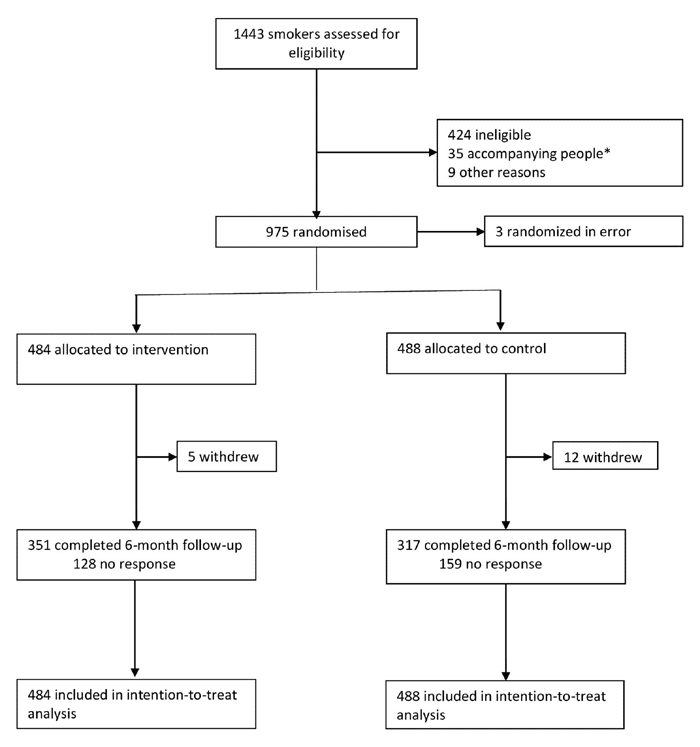
The trial was assigned to 6 UK NHS Emergency Departments (ED) and adults over 18 were chosen.
Those who reported smoking tobacco daily and attended the emergency department for medical treatment (or accompanying someone who was receiving treatment) were recruited.
There were several reasons for participants to be excluded from the study such as…
- Their Carbon Monoxide (CO) readings were less than 8 parts per million (ppm)
- Required urgent immediate medical treatment
- Were in police custody
- Known to have allergy to nicotine
- Were already vapers – even those who dual use (vape and smoke)
The eligible participants who consented to be part of the trial were split into intervention and control groups.
Control Group
This group received details of the local NHS stop smoking service via written material but were not directly referred.
Intervention Group
Those assigned to the intervention group received assistance from a dedicated smoking cessation advisor located in the ED. The assistance was…
- Brief smoking cessation advice (up to 15 minutes)
- Provision of an e-cigarette starter kit (vape) and advice on its use
- Referral to local stop smoking services.
The device chosen was the DotPro manufactured by Liberty Flights – an independent vape manufacturer with no connections to the tobacco industry.
It is a pod kit which came with 11 pods – 3x Tobacco flavour, 4x Berry flavour and 4x Menthol flavour. The pods had a 20mg Nicotine Salt strength.
Follow up support was provided by the local stop smoking service – typically by telephone offering support and advice.
Assessment
At the initial contact the participants were measured to record the baseline statistics.
Questionnaires were sent to participants 1, 3 and 6 months after their trial started.
Once completing the 6 month questionnaire the participants received a £30 shopping voucher for taking part.
Those who reported smoking abstinence at the 6 month point were invited to take a CO reading which would show their smoking status. Various methods of collecting this reading were used. If they provided the reading they were rewarded with an additional £30 shopping voucher. However this additional voucher offer was not made known to participants to avoid it acting as an incentive.
Results
Definitions
The following result definitions were used:
- Self reported (via questionnaire) and verified (by CO reading below 8 ppm) cessation of smoking at the 6 month point
- Stopping smoking for 7 days as verified by CO reading
- Number of quit attempts during the trial period
- Time between relapse
- Number of cigarettes per day
- Nicotine dependence
- Self reported dry cough or mouth / throat irritation
- Motivation to stop smoking
- Self reported use of healthcare services during the 6 months
- Self reported use of stop smoking services during the 6 months
- Quality of life
Statistics
The sample size was 972 participants (486 in each group).
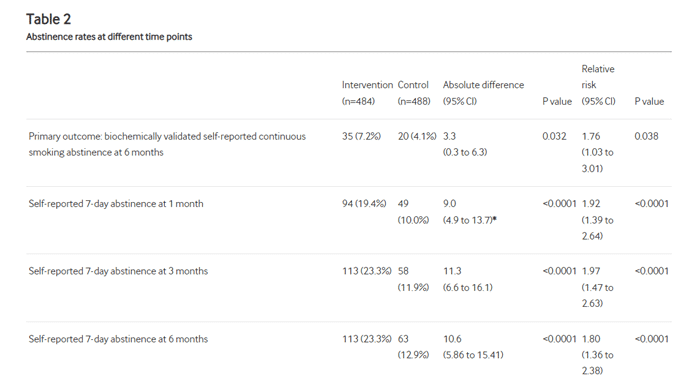
The verified quit rate at 6 months (using CO readings) were…
Control Group – 4.1%
Intervention Group – 7.2%
Also looking at the figures shows some important findings…
For instance 19.4% in the intervention group had self reported stopping smoking for at least 7 days at the 1 month follow up. Compared to 10% in the control group.
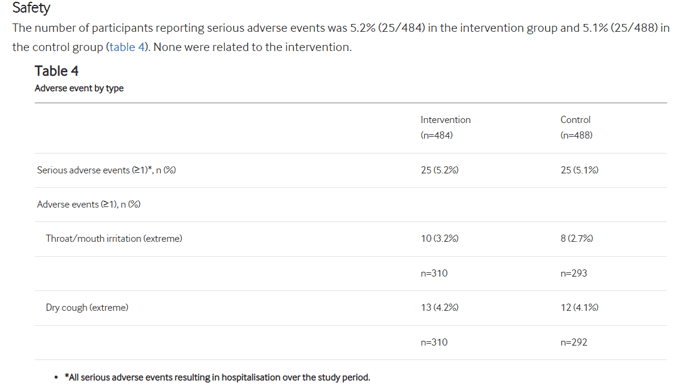
Adverse Events
Looking at the self reported adverse events data is also very interesting.
In both groups there were 25 instances of serious adverse events (resulting in hospitalisation over the study period) – none were related to the intervention. So it was equal between the vapers and non vapers.
Those in the intervention group (vapers) reported slightly higher instances of throat / mouth irritation at 3.2% than in the control group at 2.7%.
Self reporting of a dry cough was 4.2% in the intervention group and 4.1% in the control group.
As you can see there is very little difference in the results between those who vaped (intervention group) and those who quit smoking using other methods (control group).
Conclusions
I am copy pasting this as it is very important!
“In this trial adults attending the ED who smoked and received the intervention of brief advice, an e-cigarette starter kit and referral to stop smoking services were statistically significantly more likely to achieve sustained smoking abstinence than those who received signposting to stop smoking services alone. The biochemically-verified quit rate was not as high as the assumptions underpinning the power calculation; however, the difference found achieved statistical significance, with the potential to impact on population smoking prevalence. There was a much larger difference in self-reported abstinence compared with the power calculation, which may indicate that the biochemically-verified quit rate is an underestimate of the true effect of the intervention.”
“Providing smoking cessation support in the ED should be considered to reach groups of the population that may not routinely engage with stop smoking services but have the most to gain from stopping smoking.”
Also COSTED UEA have published a video telling the story of the participants in the trial…
Reactions
The SMC (Science Media Centre) published responses to the trials.
I have copied some below or you can read the full document here: expert reaction to RCT on effectiveness of ecig/quitting support offered to smokers at A&E.
Prof Lion Shahab.
Co-Director of the Tobacco and Alcohol Research Group, University College London
“This large pragmatic randomised controlled trial adds further evidence that e-cigarettes can be an effective tool for smoking cessation, extending findings to delivery in emergency rooms. Of note, this brief intervention can be easily implemented in existing healthcare delivery to make use of the time spent waiting while being seen in Accidents & Emergency. This will likely have positive health equity effects as it will reach smokers from more disadvantaged backgrounds who are more likely to attend emergency rooms.
“While absolute abstinence rates were relatively low, this probably reflects the fact that this was a very brief intervention to fit into existing treatment delivery delivered to smokers who were not necessarily thinking about quitting. Importantly, at follow-up there was no evidence of any serious adverse effects being associated with e-cigarette use, underlining their relative safety even in the context of providing them as part of acute health events that require emergency room attendance.”
Dr Sarah Jackson
Principal Research Fellow at the UCL Tobacco and Alcohol Research Group and member of the independent Trial Steering Committee for this research.
“A growing number of studies have shown e-cigarettes to be effective for helping people to quit smoking. The COSTED trial found that approaching smokers in emergency department waiting rooms and offering them a free e-cigarette starter kit (along with brief advice and referral to stop smoking services) led to a 76% increase in quitting, compared with a control condition that provided written information on stop smoking services.”
“This well-conducted trial provides further evidence that e-cigarettes can support smoking cessation. It also shows that people are receptive to smoking interventions being delivered in the emergency department: half (1443 out of 2888) of the smokers the researchers approached agreed to take part in the trial.”
“Bringing interventions like this to emergency department waiting rooms can help more people to stop smoking. It may be a particularly good way to help people from disadvantaged groups, who have high smoking rates and find it difficult to quit, but are often difficult to reach.”
Social Media Reactions
Below are a selection of reactions to the trial results.
The March episode of ‘Let’s Talk E-cigarettes’ is out here: https://t.co/CDln68298d & on @Spotify @iTunes. @jhb19 & @DrNLindson talk #vapes and interview Dr Ian Pope (@Drainpipes) about the COSTED trial (https://t.co/8tdqJLNkHb), which is hot off the press today! pic.twitter.com/6itplMooN4
— CochraneTAG (@CochraneTAG) March 27, 2024
Most people who smoke have tried to quit, but smoking is an addiction and people need support to do so🚭.The COSTED trial reveals that A&E could offer a valuable opportunity to reach people who smoke and to help them quit. See more below: https://t.co/mBY07SNJVl
— Cancer Research UK Policy (@CRUK_Policy) March 27, 2024
Great to see findings from the @CostedTrial
Vapes play a vital role in reducing harm from tobacco & research like this helps us to understand how best to target evidence based interventions.
Looking forward to the @AshOrgUK webinar this afternoonhttps://t.co/l04nGFSeoU
— North East Smokefree NHS Taskforce (@SmokefreeNHS_NE) March 27, 2024
Brief advice and a free vape kit offered to people attending an emergency department is effective for sustained smoking cessation. @drianpope @CostedTrial @UEAResearch @ueamed @HealthUEA https://t.co/fXMMiGLwaa
— AddictionResearchUEA (@AddictionUea) March 27, 2024
@CostedTrial results #SRNT2024 . Dr Pippa Belderson discussing outcomes and showing our video of participant switching stories. Opportunistic intervention in hospital EDs works for smoking cessation. @drianpope @UeaMed @UEAResearch pic.twitter.com/lNv91AKuS3
— AddictionResearchUEA (@AddictionUea) March 22, 2024
This @NIHRresearch-funded trial found that giving out free e-cigarette starter packs in A&E departments to smokers helps more people quit.
6 months after their visit, almost 1 in 4 people given the starter packs said they had quit smoking.
Read more: https://t.co/Cqrm1vx81y https://t.co/rg0ofA73BM
— NIHR CRN East of England (@NIHRCRNeoe) March 27, 2024